Specialisations for microchiropteran echolocation and moth defence co-evolution
 The suborder Microchiroptera contains approximately 800 species of bats with a sophisticated biosonar system used to actively interrogate the local environment and analyse the retuned echoes to extract information on obstacles and potential prey in the flight path. Most are insectivorous and hunt with aerial-hawking or gleaning tactics, although some, such as Noctilio leporinus, predate on fish and use echolocation to detect localised surface ripples. Much research has been conducted into the neurophysiology of echolocation with its superb acoustic discrimination capabilities, including very fine temporal and frequency resolution. This review covers firstly bat call design and hunting behaviour, and the neural basis of their capabilities. The second half deals with the question of a bat-moth co-evolutionary arms race.
The suborder Microchiroptera contains approximately 800 species of bats with a sophisticated biosonar system used to actively interrogate the local environment and analyse the retuned echoes to extract information on obstacles and potential prey in the flight path. Most are insectivorous and hunt with aerial-hawking or gleaning tactics, although some, such as Noctilio leporinus, predate on fish and use echolocation to detect localised surface ripples. Much research has been conducted into the neurophysiology of echolocation with its superb acoustic discrimination capabilities, including very fine temporal and frequency resolution. This review covers firstly bat call design and hunting behaviour, and the neural basis of their capabilities. The second half deals with the question of a bat-moth co-evolutionary arms race.
Signal design & the hunting sequence
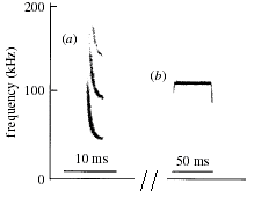
Bat echolocation functions by emitting ultrasonic-frequency (20-200KHz4) pulses focused, often by specialised nasal structures, into a highly directional, narrow (60°) cone. Two basic acoustic forms are used: broadband frequency modulated (FM) sweeps of about 5ms duration and longer narrowband constant frequency (CF) signals, as seen in Figure 1.
Figure 1. (a) downward FM sweep with strong harmonics of Pipistrellus pipistrellus. (b) FM/long CF/FM echolocation signature of Rhinolophus hipposideros. Adapted from Jones et al (2000)2
The most prominent frequency and the signal signature varies between species, and may consist of either an FM or CF pulse, or a combination of the two. CF signals are well suited for long-range detection of objects, as pure tones carry more energy than FM sweeps and consequently propagate further before attenuating below the auditory threshold. They are also suitable for measuring the Doppler shift of the returned echo which can potentially provide two pieces of information about the target: the approach speed (steady shift), and in the case of insect prey, the frequency of wing flapping (periodic shift). The former allows the bat to adjust its attacking run or gently approach a perch, whilst the later aids discrimination of insect echoes from inedible clutter such as leaves. During the beat cycle the wing surfaces regularly approach and recede from the bat, producing a frequency up-shift and down-shift respectively, as well as intensity variations; the periodicity of which is neurally computed. Surface textures imprint on the echo a characteristic “colour”, and so FM sweeps are potentially very good at target description. FM sweeps also allow many estimates of the pulse-echo delay, and hence target range, from the same pulse. The bandwidth of FM sweeps (especially with harmonics) thus aids target description and accurate ranging, whilst the forte of CF tones is in assessing relative velocities and initial prey detection, both through its greater detection range and sensitivity to periodic intensity fluctuation and Doppler shift. The greater detection range of CF pulses is only an asset in open areas, whilst the target-discrimination ability of FM sweeps is preferable in crowded, acoustically cluttered environments, such as around vegetation or the ground. CF bats can however compensate in cluttered situations by relying on the motion of a moth through successive echoes5. Signal design is thus tailored to the local ecology and information requirements of the hunting bat.
Three phases have been identified in the standard routine of bats during prey interception, obstacle avoidance and landing: search, approach and terminal. The search phase is characterised by a slow repetition rate (“low duty cycle”) of about 10s-1. Experiments performed on Eptesicus fuscus suggest it can detect a 2cm diameter sphere at a distance of 5m4. A bat under field conditions however first reacts to a flying insect at a much lesser range, although this may represent a critical reaction distance rather than a detection threshold. Once a potential target has been identified, the bat vectors towards it and initiates the approach phase at about 2m distance. During the approach phase the duty cycle is increased to a maximum of about 40s-1, whilst the duration of each pulse is often shortened to avoid overlap between emitted pulses and echoes. Species that utilise FM sweeps, such as Myotis myotis, increase the sweep gradient to maintain the bandwidth and also shift to lower frequencies. In general, during the approach phase brief broadband FM sweeps are favoured and CF components are diminished, as this focuses on target characterisation and range calculation. As the bat closes to about 0.5m the terminal phase is activated and the repetition rate is abruptly increased to up to 200s-1: the “terminal buzz”. Almost all microchiropterans rely solely on very brief (0.5ms) FM sweeps at this stage to pinpoint the prey’s position to within about a cubic centimetre4 and scoop it up in outspread wing membranes before being eaten.
Neurophysiology of echolocation
In addition to perception of extrinsic sounds and conspecific communication signals (such as mating calls) and mammalian adaptations for elevation and azimuthal localisation of sound, bats have developed faculties for extremely high resolution in the temporal and frequency domains. Sound localisation depends on perception of inter-aural timing, intensity, and phase differences in the binaural pathways, whereas bat specialisations are mostly found in the monaural pathways before convergence. This review will concentrate on these extra developments required for accurate echolocation, as mammalian synapomorphies are covered by any standard textbook, such as Bear et al (1996)6.
In long-CF employing species the portion of the basilar membrane just higher than the principal emitted frequency (not necessarily the fundamental) is disproportionately represented. In R. ferrumequinum the narrow band between 83 and 84 KHz, where Doppler shifted echoes lie, constitutes 8% of the total number of spiral ganglia4, and is known as the acoustic fovea. Phyllostomus hastatus channels most of the energy emitted in its long-CF/short-FM call into the 2nd harmonic of around 61KHz, which is again over-represented in the cochlea7. Such species modify the principal frequency on approach to a target to ensure that the echo always falls onto this fovea; a technique called Doppler shift compensation. The auditory threshold is lowest (greatest sensitivity) at these frequencies, but much higher at the actual emitted frequency so that the bat does not swamp the auditory system with its own high intensity calls. Species with prominent FM sweeps have a much broader minimum in the threshold curve.
The auditory periphery contains only a cochleotopic (frequency dimension) map, and does not encode amplitude or timing (neither duration nor periodicity) domains. This information is computationally extracted at higher levels in the auditory pathway7. Like the mammalian visual system6, bat audition relies on parallel processing, with distinct information streams encoding different elements, and more complex functions built up by ascending pathway convergence in higher levels of the hierarchy. The three most important structures in the echolocation-processing pathway are the cochlea nucleus (brainstem), inferior colliculus (midbrain) and auditory cortex (forebrain). There also exists a descending “corticofugal” pathway which projects from the auditory cortex back to the cochlea.
Ascending pathway
Cochlear Nucleus (CN)
Neurones in this region of the brainstem have been found to be active for the entire period that a sound of particular frequency is above its threshold (indiscriminate with regards to intensity), and thus mark the duration of a pulse8. Histochemical staining for glycine and GABA suggests that a major function of the CN is to provide inhibitory input to the IC9 which is crucial for the building of more complex feature detectors. Spiking latencies are greater in the CN than the cochlea, and in fact latency increases by an order of magnitude in the pathway from CN (about 4ms) to IC (up to 40ms9). Thus the CN serves to transform output from excitatory to inhibitory, modify the temporal patterns of neural discharge, and create delay lines9. Information from the CN passes via the lateral lemniscus to the inferior colliculus.
Inferior Colliculus (IC)
Further convergence produces frequency-tuned neurones that generate a single spike on the onset or offset of a pulse (so called time-markers). Other neurones are tuned to duration of pulse8, direction of FM sweep, and rate of periodic modulation that function to filter extraneous noise from biologically important sounds8. For example, the search phase calls of E. fuscus are 20ms, 30KHz CF pulses and are detected among ambient noise by neurones with best duration of 20ms, frequency-tuned to 30KHz. Sinusoidal FM-sensitive neurones have been identified in E. fuscus9, and probably play a role in perception of wing fluttering-induced Doppler shifts. Models of the mechanism of the above detectors have been proposed, as reviewed in Covey et al (1999)9. For example, the construction of a pulse-duration-tuned neurone is believed to be dependent on the inhibitory inputs and latencies generated in the CN, as inhibitory neurotransmitter antagonists block duration timing. The tuned neurone receives two inputs, one inhibitory for the duration of the signal followed by a post inhibitory rebound marking the offset, and the other a delayed excitation triggered by the sound onset. Only when the two excitatory inputs temporally coincide does summation exceed the threshold and produce a spike. The latency value of the second excitation is fixed and intrinsic to the neurone, and determines the neurone’s best-duration.
The isofrequency slabs of the tonotopic map in P. parnelli have been found to each contain a dorso-ventral latency axis, which is thought to be involved in the creation of delay-lines used in the range-finding functions of the FM-FM region of the AC10. Range calculation based on pulse-echo delays is also dependent on time-marking neurones in the IC being able to recover quickly after registering the emitted pulse and spiking again on the echo. Such neurones in most mammalian auditory systems require around 10ms to recover, whereas bat neurones respond to the second sound even if it occurs within 1ms of the first. Furthermore, the response latency of such bat neurones is independent of stimulus intensity, unlike the inverse proportionality of other mammals, and thus minimises coding-error due to the different amplitudes within pulse-echo pairs4. Such fine temporal resolution may well represent the most fundamental adaptation required for the evolution of echolocation.
Auditory Cortex (AC)
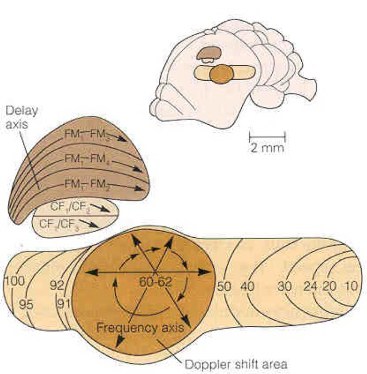
The AC of P. parnelli, as seen in Figure 2, is well studied, and is where further convergence occurs to produce combination-sensitive neurones; target range, relative velocity and modulation periodicity are coded for in distinct cortical maps.
Figure 2. Diagram of cerebral hemisphere, and detailed schematic of AC of P. parnelli showing DSCF, FM-FM and CF-CF areas. Taken from Kandel and Schwartz (2000)1
The Doppler shifted constant frequency (DSCF) area (acoustic fovea) contains overlapping orthogonal frequency and amplitude maps, resulting in neurones coding for the periodicity of insect wing beats. The CF-CF region (CF1/CF2 and CF1/CF3 areas) contains neurones encoding the Doppler shift between the emitted pulse and echo of the 2nd and 3rd harmonics respectively. The neurones are sharply frequency tuned and fire in response to a specific target relative velocity7, with delays representing 0 – 4ms-1 over-represented as they are especially important during landing and prey interception. Dorsal of this region is the FM-FM area, with FM1-FM2, FM1-FM3 and FM1-FM4 subdivisions containing neurones combination-tuned to best-frequencies and best-delays between the emitted call and 2nd, 3rd and 4th harmonic echoes respectively. The FM-FM area thus constitutes three echo-delay, or target range, maps. The auditory cortex therefore contains multiple cochleotopic maps, and comprises the culmination of convergent information streams with its computational maps of amplitude, velocity and range.
Descending pathway
The corticofugal projection modulates information processing7 in the ascending system by narrowing the duration- or frequency-tuning curves of subcortical neurones through positive feedback loops and lateral inhibition 11.
Moth Defences
The predation risk presented by echolocating bats would be expected to have exerted a significant selective pressure on prey for the evolution of ultrasonic perception and avoidance strategies. In fact six insect orders have acquired the capability to detect echolocation signals2, most notably the noctuid moths with tympanic “ears” sensitive to ultrasonic frequencies12. Noctuids insensitive to bat echolocation calls have been shown to have a greater predation rate13, and diurnal moths no longer exposed to bats exhibit a state of advanced auditory degeneration14. The noctuid auditory system is most sensitive to an intense12, 15, mid-frequency (20-50KHz) long (5-10ms) CF pulse, and under these conditions can detect an echolocating bat at a range of around 30m. Detection triggers a bimodal anti-bat response, eliciting distinct behavioural countermeasures dependent on the situation. Each tympanic membrane is enervated by two sensory neurones; A1 and A2. A1 is the more sensitive of the two, and when activated triggers co-ordinated flight in a vector that projects away from the source (phonotactive flight) in order to escape the bat’s flight path before it comes within perception range12. A2 has a much higher threshold (by about 25dB12), and so is only activated when the bat is much closer and its echolocation signals more intense. A2 triggers erratic excitation or inhibition of the longitudinal flight muscles, and so causes complex flight manoeuvring of rapid dives and turns in an attempt to escape the highly directional, narrow echolocation cone. Wing flapping is also episodically ceased to reduce the moth’s apparency to the bat’s auditory system (especially the periodic modulation detectors), causing its echo signature to resemble an inedible leaf more than a prey item. The A2 acoustic startle response is ballistic and un-modifiable once activated and so triggering such behaviour could be very risky, for example when the moth is flying low over water. A1 and A2 induced responses are termed primary and secondary defences, as they respectively occur before and after a predator attacks them (definition by Edmunds, 1974).
Waters (1996)12 has analysed the information coding capabilities of the A1 neurone, and found it to be a particularly unreliable indicator of the presence or absence of a bat with low intensity and short duration calls. It is possible microchiropterans and noctuids are engaged in an arms race, with bats striving to render themselves less detectable and moths co-evolving more sensitive auditory systems.
Gleaning, or “whispering”, bats such Phyllostomus hastatus, appear to be particularly well adapted to stealth attacks. They have especially broad wings (low wing load) to enable quiet, slow flight over an area of ground as the bat passively listens for incidental sounds created by prey. Short duration, low intensity echolocation calls in a low duty cycle, or even with irregular repetition may be used, but are invariably switched off before terminal phase to avoid alerting the prey. Greater intensity calls with higher duty cycles are still used for flight navigation, and some species, Myotis septentrionalis for example, switch between both gleaning and aerial-hawking hunting modes16, 12, 17. Adoption of constant frequencies beyond the response envelope of the tympanic ear, so called allotonic signals, is also common among bats. Many rhinolophoids for example emphasise the 2nd harmonic (typically at around 80KHz) which is above the audible range of moths2. This signal design can only be definitely attributed to a co-evolutionary arms race however if higher frequency signals did not evolve for other reasons, such as greater directionality, better detectability of smaller targets, or a channel free of other bat calls. A better candidate for co-evolution is the implementation of low frequency (<15KHz) allotonic calls by Tadarida teniotis18, which in fact incurs a cost of reduced reflectance off moths2. The potential for evolving allotonic calls is greatly reduced in species employing broadband FM sweeps.
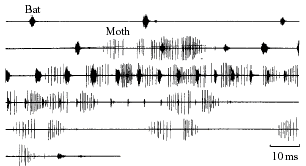
Arctiid moths have a further adaptation that reduces their susceptibility to bat predation. In response to high frequency sounds Cycnia Tenera undergoes the familiar sequence of erratic flight and dropping, but then generates a series of rapid, ultrasonic, variable clicks (as seen in Figure 3) with a pair of thoracic tymbals under closed-loop control19 that causes the bat to break off the attack. It is not known however whether this click train serves as an aposematic warning, informing the bat of its indelectability, or to startle, or even functions to disorientate the predator’s auditory system. This latter “jamming” function may operate by creating a phantom echo of an object in the flight path that the bat steers to avoid, or severely interfering with prey range-finding20, 21. Irregularly spaced, variable-profile clicks improve the chances that some will closely acoustically mimic a real echo and create a phantom perception3. Fullard (1994)3found that the phonoresponse is invariably emitted during the terminal phase, which would maximise disorientation, rather than as soon as the moth detects the bat’s presence, as would be expected for an aposematic signal aimed at providing information to the bat during its attack decision.
Figure 3.The irregular repeated, variable click train of C. tenera coincides with the terminal buzz of E. fuscus. Adapted from Fullard et al. (1994)3
Conclusions
This review has discussed the vocalisation and auditory system specialisations developed by microchiropterans in their echolocation system for navigation and hunting. The signal design has constant frequency and/or frequency-modulated components tailored to the ecology of the hunting grounds, which provide information on different aspects of objects illuminated by the directional sonar cone. Temporal and frequency modifications correlate with the bat progressing through search, approach and terminal phases. Distinct ascending information streams encoding different acoustic parameters converge in higher levels of the parallel processing hierarchy to produce combination-sensitive neurones arranged in computational maps. Complex cortical feature detectors responding to target velocity, range and periodic modulation are thus extracted from the basic cochleotopic map using inhibitory and latency transformations. The corticofugal pathway acts through positive feedback loops and lateral inhibition to narrow subcortical tuning curves. Adaptations unique to microchiropterans among terrestrial mammals (although echolocating dolphins make have similar specialisations) include the acoustic fovea, and rapid recovery and constant latency of time-marking neurones.
There exists good evidence13,14 that noctuid tympanic ears and the bimodal escape behaviour have evolved in response to the predation pressure exerted by echolocating bats. The adaptations of gleaning bats and those employing signals with frequencies lower than the auditory envelope of the moths are almost surely a result of co-evolution with the noctuids or other hearing prey. It is less certain however whether high-frequency allotonic signals evolved in response to moth audition, or for some other purpose, such as improved directionability, and thus may not constitute a co-evolutionary adaptation.llotonic signals evolved in response to moth audition, he ls higher than the Arctiid moths have additionally developed ultrasonic click trains, although it is uncertain whether these serve mainly an aposematic, startle, or jamming function (either by creating a phantom echo or disrupting range-finding). Fullard (1994)3 suggests that the click train is designed to cause maximum auditory disruption and thus serve a jamming role, although it should be noted that other studies have produced contradictory results. Either way, a moth is likely to encounter several different bat species and other predators, and so the defence mechanism cannot be expected to be perfectly optimised to one specific situation. If arctiid clicks do serve a primarily jamming rôle it is possible that bats may evolve resistance through improved echo-signature discrimination mechanisms.
Bibliography
- Kandel, E., Schwartz, J. & Jessell, T. Principles of Neural Science 4th Ed. (2000).
- Jones, G. & Waters, D. A. Moth hearing in response to bat echolocation calls manipulated independently in time and frequency. Proc R Soc Lond B Biol Sci 267, 1627-32 (2000).
- Fullard, J. H., Simmons, J. A. & Saillant, P. A. Jamming bat echolocation: the dogbane tiger moth Cycnia tenera times its clicks to the terminal attack calls of the big brown bat Eptesicus fuscus. J Exp Biol 194, 285-98 (1994).
- Young, D. Nerve Cells and Behaviour (Cambridge University Press, 1996).
- Jensen, M. E., Miller, L. A. & Rydell, J. Detection of prey in a cluttered environment by the northern bat Eptesicus nilssonii. J Exp Biol 204, 199-208 (2001).
- Bear, M., Connors, B. & Paradiso, M. Neuroscience: Exploring the Brain (Williams & Wilkins, 1996).
- Suga, N., Yan, J. & Zhang, Y. Cortical maps for hearing and egocentric selection for self-organization. Trends in Cognitive Neurosciences 1, 13-20 (1997).
- Ehrlich, D., Casseday, J. H. & Covey, E. Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol 77, 2360-72 (1997).
- Covey, E. & Casseday, J. H. Timing in the auditory system of the bat. Annu Rev Physiol 61, 457-76 (1999).
- Hattori, T. & Suga, N. The inferior colliculus of the mustached bat has the frequency-vs-latency coordinates. J Comp Physiol [A] 180, 271-84 (1997).
- Zhang, Y., Suga, N. & Yan, J. Corticofugal modulation of frequency processing in bat auditory system. Nature 387, 900-903 (1997).
- Waters, D. The peripheral auditory characteristics of noctuid moths: information encoding and endogenous noise. J Exp Biol 199, 857-68 (1996).
- Fullard, J. H. Auditory sensitivity of Hawaiian moths (Lepidoptera: Noctuidae) and selective predation by the Hawaiian hoary bat (Chiroptera: Lasiurus cinereus semotus). Proc R Soc Lond B Biol Sci 268, 1375-80 (2001).
- Fullard, J. H., Dawson, J. W., Otero, L. D. & Surlykke, A. Bat-deafness in day-flying moths (Lepidoptera, Notodontidae, Dioptinae). J Comp Physiol [A] 181, 477-83 (1997).
- Waters, D. A. & Jones, G. Echolocation call structure and intensity in five species of insectivorous bats. J Exp Biol 198 ( Pt 2), 475-89 (1995).
- Fullard, J. & Dawson, J. The echolocation calls of the spotted bat Euderma maculatum are relatively inaudible to moths. J Exp Biol 200, 129-37 (1997).
- Faure, P. A. & Barclay, R. M. Substrate-gleaning versus aerial-hawking: plasticity in the foraging and echolocation behaviour of the long-eared bat, Myotis evotis. J Comp Physiol [A] 174, 651-60 (1994).
- Rydell, J. & Arlettaz, R. Low-frequency echolocation enables the bat Tadarida teniotis to feed on tympanate insects. Proc R Soc Lond B Biol Sci 257, 175-8 (1994).
- Northcott, M. A. & Fullard, J. H. The closed-loop nature of the tymbal response in the dogbane tiger moth, Cycnia tenera (Lepidoptera, Arctiidae). Brain Behav Evol 48, 130-6 (1996).
- Miller, L. A. Arctiid moth clicks can degrade the accuracy of range difference discrimination in echolocating big brown bats, Eptesicus fuscus. J Comp Physiol [A] 168, 571-9 (1991).
- Tougaard, J., Casseday, J. H. & Covey, E. Arctiid moths and bat echolocation: broad-band clicks interfere with neural responses to auditory stimuli in the nuclei of the lateral lemniscus of the big brown bat. J Comp Physiol [A] 182, 203-15 (1998).
Species mentioned in this review:
Eptesicus fuscus – big brown bat
Myotis myotis – mouse-eared bat
Myotis septentrionalis – eastern long-eared myotis
Noctilio leporinus – greater bulldog bat
Nyctalus noctula – noctule bat
Pteronotus parnelli – moustached bat
Phyllostomus hastatus – spear-nosed bat
Pipistrellus pipistrellus – common pipistrelle
Rhinolophus ferrumequinum – greater horseshoe bat
Rhinolophus hipposideros – lesser horseshoe bat
Tadarida teniotis – European free-tailed bat
Cycnia Tenera – dogbane tiger moth